Digital Risk Management for Advanced Medical Devices call for Digital Risk Management


Designing a medical device is not an easy task. It requires careful documentation and collaboration, as there is no margin of error and, at the end of the day, your Medical Devices are as good as your Design Controls.
A strong risk management will help you identify hazards and hazardous situations which will feed into the Design Controls process when you define User Needs and Design Inputs.
And better than a strong risk management, it's a digitalised risk management using a centralised platform.
iRISK™ will optimise your risk management by streamlining your operations, improving your reporting, and making a more efficient use of your resources.
We understand that you need to have a clear picture of when would iRISK™ start making your Risk Management so much easier. Our deployment process is very streamlined, which is why we can predict that a standard installation will take approximately one month.
You should keep in mind that, if a customised iRISK™ better fits your needs, these installation timelines will be adjusted and agreed with you based on the extent of the required customisations.
Our team will make sure the system works properly and that your other systems are perfectly integrated iRISK™.
At this point, we will deliver a carefully planned training to your team to make sure you can make the most out of iRISK™.
It's Go-Live time! This also marks the start of the one month Hypercare phase where we will support closely your iRISK™ use.
Our team will make sure the system works properly and that your other systems are perfectly integrated iRISK™.
At this point, we will deliver a carefully planned training to your team to make sure you can make the most out of iRISK™.
It's Go-Live time! This also marks the start of the one month Hypercare phase where we will support closely your iRISK™ use.
iRISK™ is licensed through an annual subscription for each user. User License price decreases as the total of purchased User Licenses increases.
iRISK™ includes upgrades and fixes, as well at least 5h per year, per User License of Remote Support.
iRISK™ can be deployed at a client's infrastructure/cloud or using 4TE cloud provider (Microsoft Azure).
iRISK™ is your platform. This means that it will be adapted to your organisation's unique requirements so that you can maximize the potential of your team's performance.
The expertise of our team when dealing with most data sources means that iRISK™ can be integrated with virtually any 3rd party software.
We will load all your historical data and templates into iRISK™. This means that you will get only benefits from adopting a new tool and none of the friction.
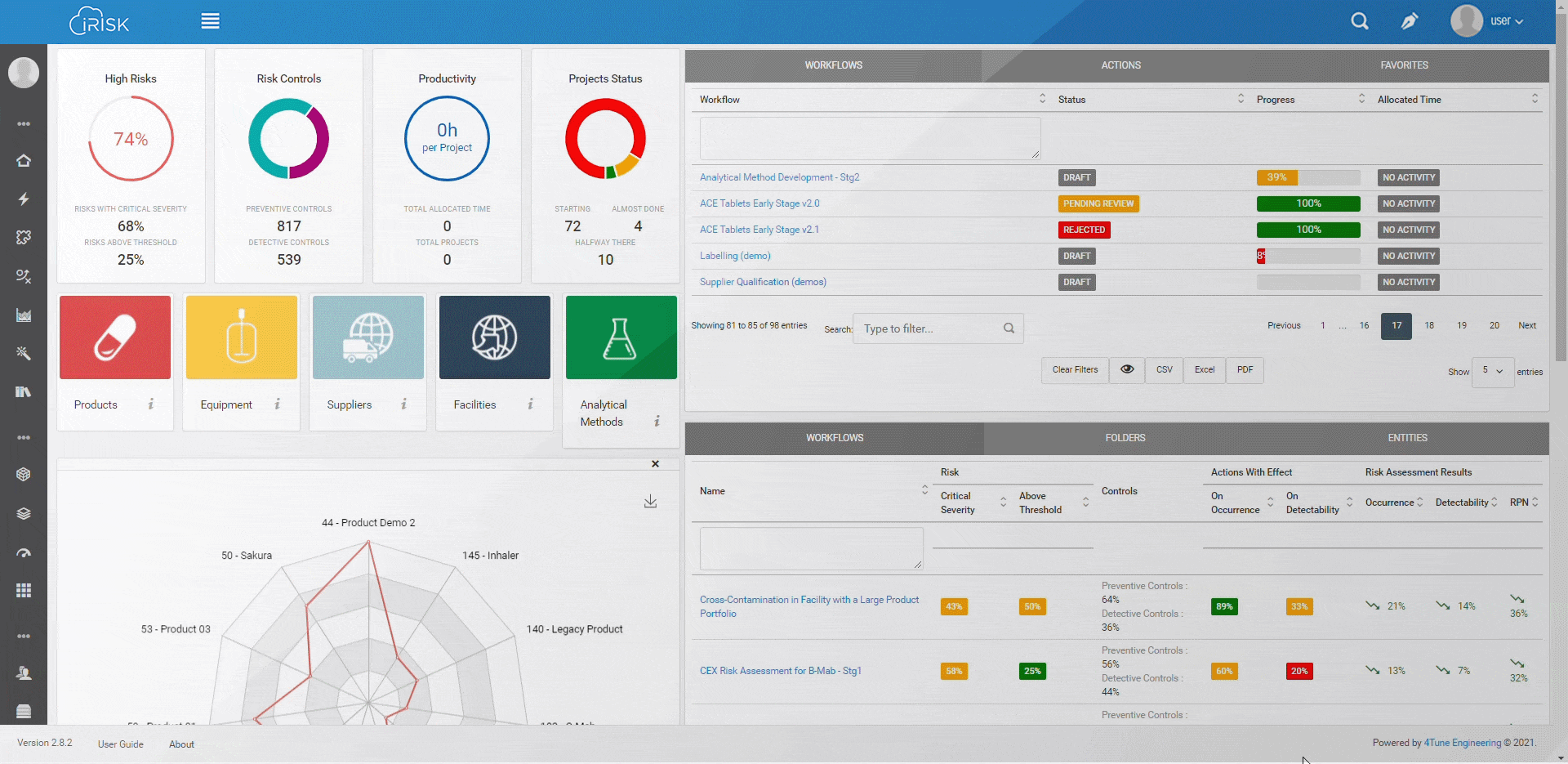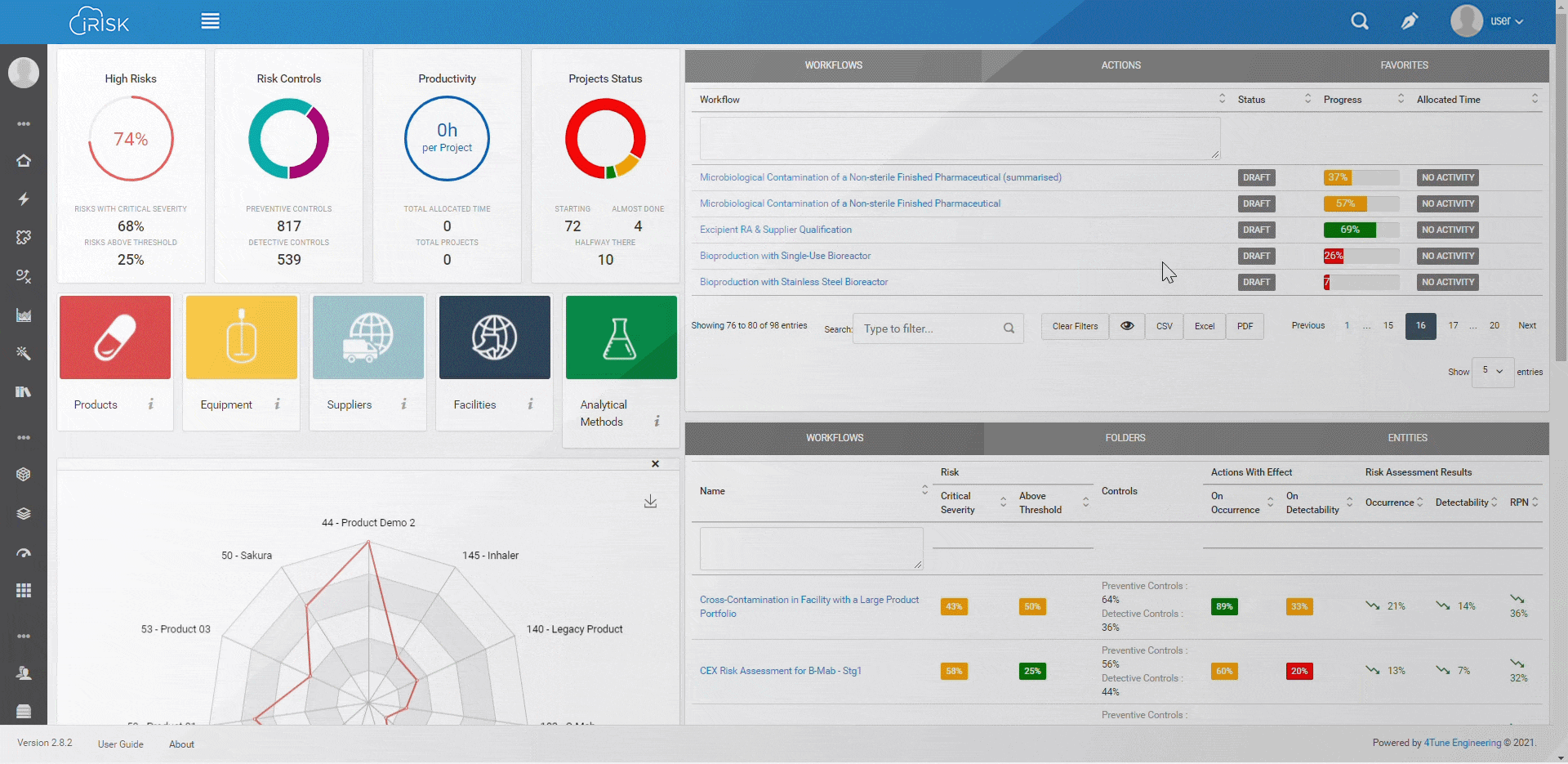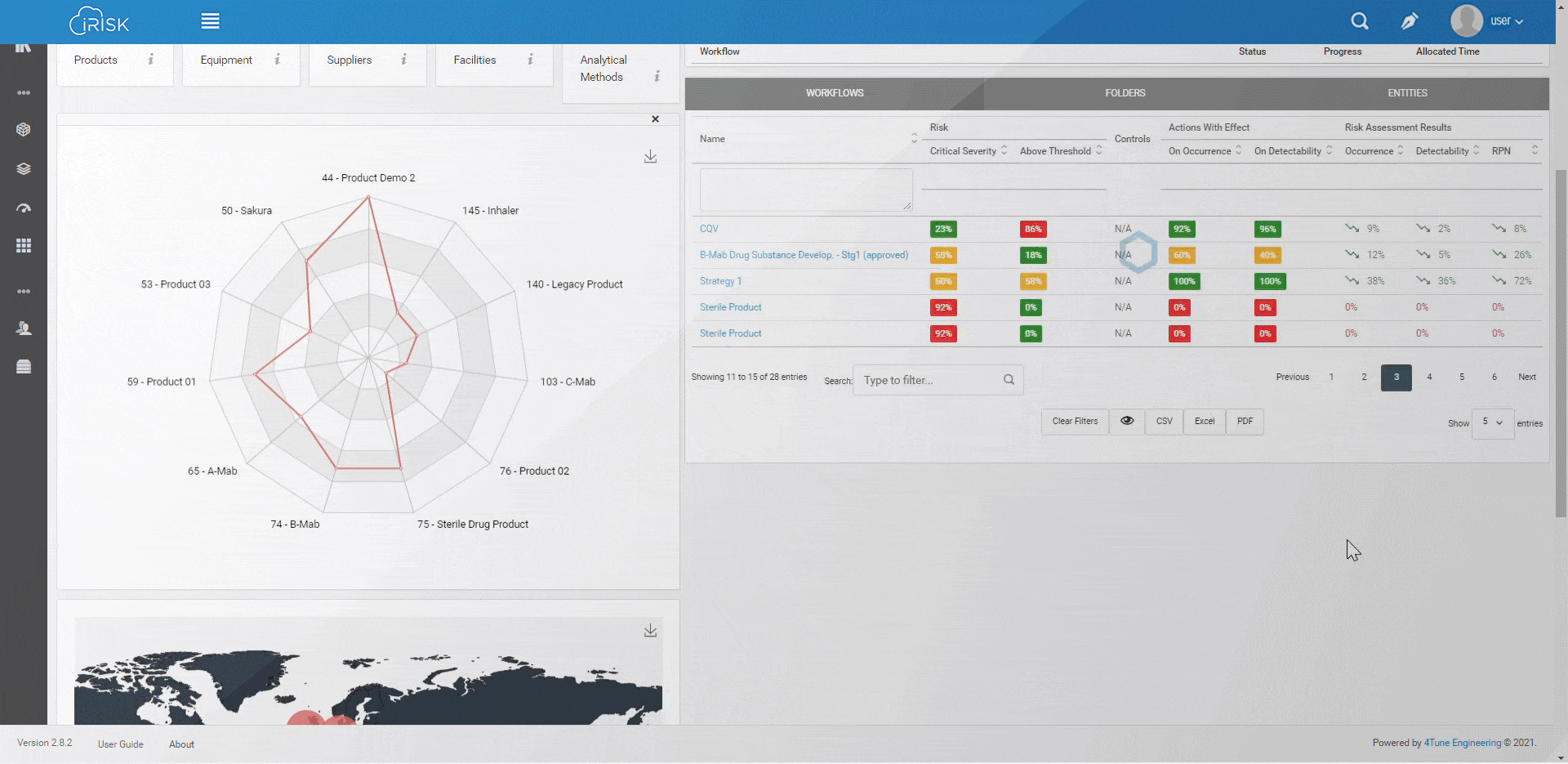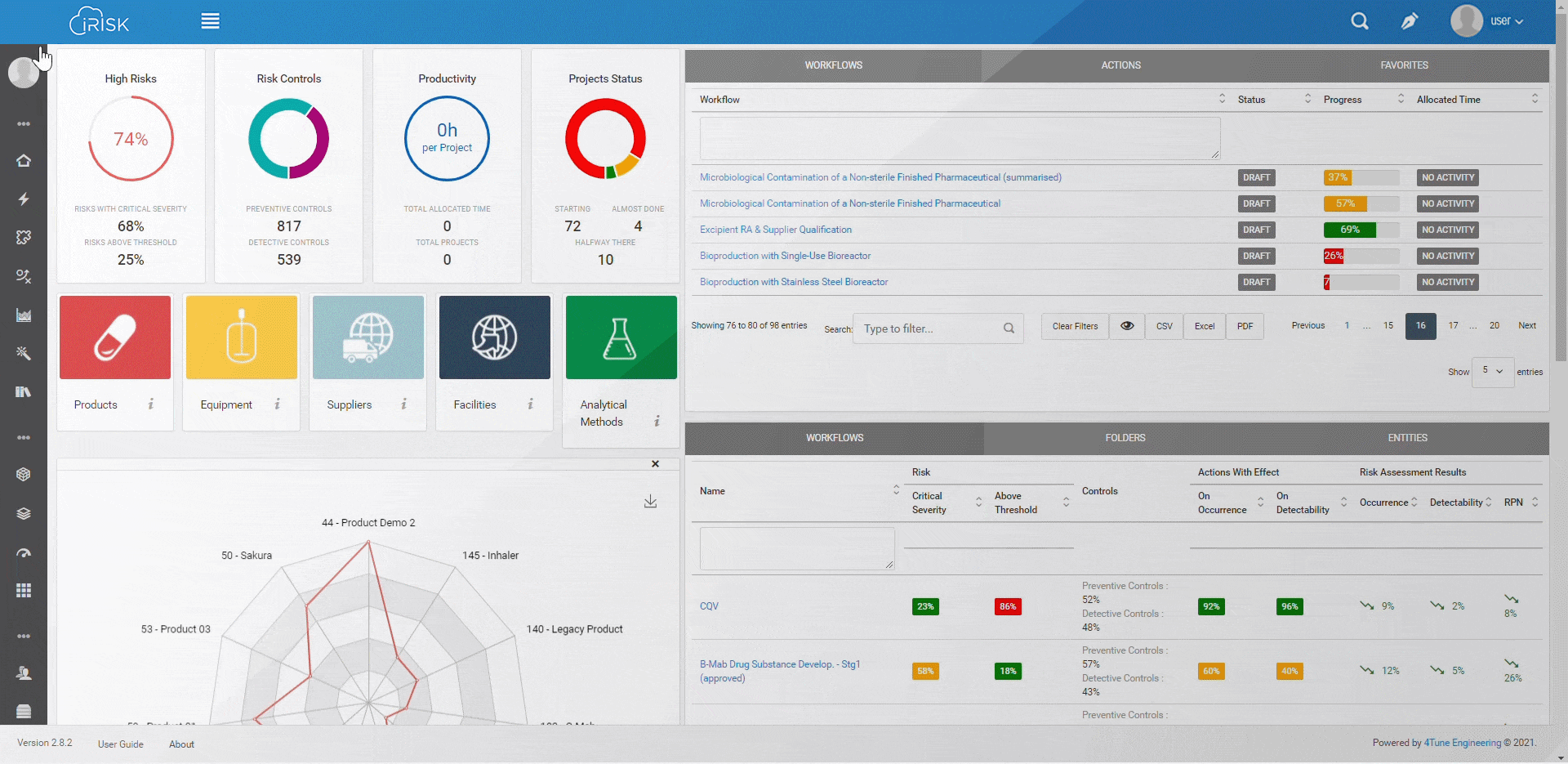
iRISK™ builds traceability in the risk management of your medical device development process. Each stage of your product lifecycle is covered: process, production and post-market use.
More than having a platform that helps you identify, quantify, and prioritise the risks, you must assess and link operations to patient outcomes with an approach that aggregates evidence and extracts insights for science-based decisions.
Assure quality compliance during the whole manufacturing process to guarantee product efficacy and patient safety. iRISK is the platform that gives you tools to make informed choices about compared risk and is the basis to support your decision-making process.
But how can you make this happen? Our team will give you full support to ensure that you make the most out of risk management digitalisation.